Embryo Freezing
- /
- Embryo Freezing
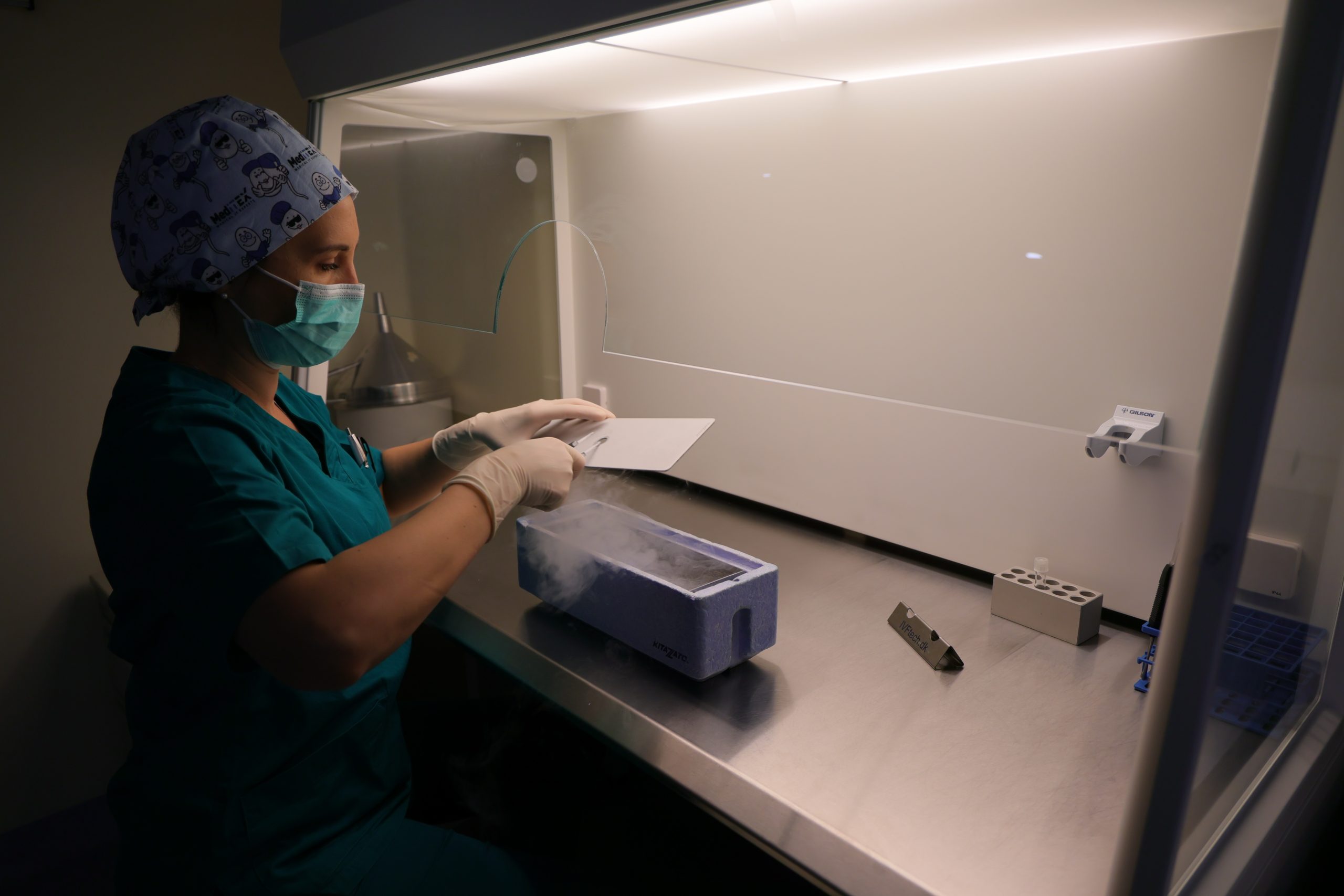

Cryopreservation
Embryo cryopreservation (freezing) is offered to patients who have a high number of normally fertilized embryos or high-quality blastocysts remaining after the embryo transfer. Embryos can be frozen either on the third day after egg retrieval or on the 5th or 6th day at the blastocyst stage.
Freezing can be performed at any stage, and the embryos can be preserved for several years at sub-zero temperatures using liquid nitrogen. These embryos can later be thawed and transferred to the patient during a frozen embryo transfer cycle (FET).
STAGES
- It involves stimulating the ovary (or ovaries) to produce multiple follicles and consequently as many eggs as possible (IVF). During the stimulation phase, follicular growth is carefully monitored through transvaginal ultrasound, while estradiol and luteinizing hormone (LH) levels in the blood are simultaneously tested. These are hormones produced by the follicles as they grow and help determine the optimal timing for egg retrieval.
- When a sufficient number of follicles have reached satisfactory growth levels, final oocyte maturation is triggered with a dose of hCG.
- The next step is the retrieval of the eggs from the follicles. This is performed using an aspiration needle through the vaginal wall, guided by ultrasound imaging. The procedure is done under deep intravenous anesthesia. Typically, the patient can leave the clinic about 30–60 minutes after the procedure.


After the Procedure:
A few hours after the procedure, oocytes that show an appropriate degree of maturity (metaphase II) are cryopreserved (through vitrification) and stored in liquid nitrogen.
The vitrification process consists of three main components. First, the eggs or embryos are exposed to high concentrations of cryoprotectants to allow rapid dehydration of the cells. Second, the eggs or embryos are loaded into storage devices (cryotops) that facilitate ultra-rapid cooling. Third, the cryotops containing the eggs or embryos are plunged into liquid nitrogen as quickly as possible.
In practice, this means that the eggs and embryos are vitrified very quickly in the laboratory. A complete embryo vitrification protocol takes about 10 minutes. The embryos are removed from the incubator and initially exposed to an equilibration medium for 8 minutes to begin the dehydration process. They are then placed for 60 seconds in a second solution, where the media differ in the concentration of their components. Next, they are rapidly loaded onto a cryotop and immersed in liquid nitrogen at a temperature of -196°C. The cryotop is cooled from room temperature (approximately 25°C) to -196°C in 2–3 seconds, achieving a cooling rate between 4420 and 6630°C per minute.
This extremely high cooling rate, combined with the use of high concentrations of cryoprotectants, turns the contents of the cryotop (embryos plus surrounding medium) into a glass-like state rather than ice. Successfully avoiding ice crystal formation protects the embryos from damage and allows them to be safely warmed for future use, with a post-thaw survival rate of over 90%.
When patients return to use their vitrified embryos or eggs, the vitrification procedure described above is reversed, allowing the warming and gradual return of the eggs or embryos to room temperature, and then maintaining them in culture at 37°C, while simultaneously rehydrating them. The eggs and embryos are not technically frozen — no ice forms inside the cells. The warming (“thawing”) process takes only about 20 minutes, after which the eggs or embryos are placed back in the incubator at 37°C. Embryos can then be transferred to the uterus immediately, while eggs can be injected, each with a single sperm cell, about 3–4 hours later.
